🧠 Introduction: Entropy क्या होती है?
Thermodynamics में Entropy (एंट्रॉपी) एक बहुत important concept है।
Simple words में कहें तो entropy किसी system के disorder या randomness को represent करती है।
जितनी ज्यादा randomness, उतनी ज्यादा entropy!
Example:
- Ice (बर्फ) में molecules ordered रहते हैं → low entropy
- Water में molecules random होते हैं → high entropy
इसका मतलब जब system disorder की तरफ बढ़ता है, तो entropy increase होती है।

📘 Entropy Definition
In thermodynamics, entropy is defined as the measure of randomness or disorder of a system.
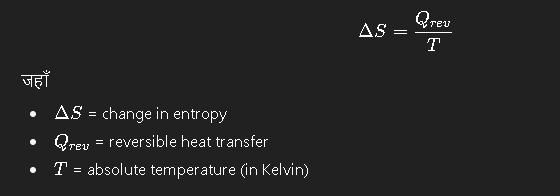
Mathematically इसे इस formula से represent किया जाता है:
⚙️ Entropy का Physical Meaning
Entropy हमें बताती है कि energy किस हद तक usable है।
जब भी energy convert होती है (जैसे heat से work), तो उसकी कुछ मात्रा loss हो जाती है as unusable energy — यही entropy का effect है।
👉 Simple example:
अगर 100% energy से काम करना possible होता, तो entropy zero होती।
लेकिन practically ऐसा कभी नहीं होता, इसलिए हर real process में entropy हमेशा बढ़ती है।
📈 Laws of Thermodynamics and Entropy
1️⃣ Second Law of Thermodynamics:
It states that the entropy of an isolated system always increases with time.
यानी किसी closed system में natural processes हमेशा high entropy direction में चलते हैं।
2️⃣ Reversible Process:
Entropy constant रहती है (ΔS = 0)
3️⃣ Irreversible Process:
Entropy बढ़ती है (ΔS > 0)
📏 Unit of Entropy
- SI Unit: Joule per Kelvin (J/K)
- CGS Unit: Calorie per Kelvin
🔍 Example to Understand Entropy
Imagine you have a perfume bottle.
जब आप उसे open करते हैं, perfume molecules पूरे room में फैल जाते हैं — यह process irreversible है और entropy बढ़ती है क्योंकि system का disorder बढ़ गया।
💡 Importance of Entropy in Engineering
- Helps in analyzing heat engines and refrigeration cycles
- Predicts efficiency of thermodynamic systems
- Explains irreversibility in natural processes
🧩 Summary
| Property | Description |
|---|---|
| Symbol | S |
| Unit | J/K |
| Meaning | Measure of disorder |
| Increases in | Irreversible processes |
| Constant in | Reversible processes |
🎯 Conclusion
Entropy बताती है कि nature हमेशा order से disorder की तरफ move करता है।
It’s one of the most important topics in thermodynamics that helps engineers understand how energy is used and lost in real systems.
📚 Related Topics
- What is Enthalpy?
- Second Law of Thermodynamics Explained
- Carnot Cycle and Entropy Relation




Leave a Reply